Navigating leather care can be confusing due to the sheer volume of misinformation online. The leather experts at Advanced Leather Solutions created this FAQ section to cut through the noise with answers based strictly on the Science of Leather, not myths.
If you don't see your specific question listed below, don't guess—ask the expert. I personally invite you to reach out for advice tailored to your gear.
Email me at Kevin@AdvLeather.com, and I will respond with the scientific facts you need to protect your investment.
Frequently Asked Questions
Why is it important to have the correct pH of baseball glove care products like cleaners and conditioners?
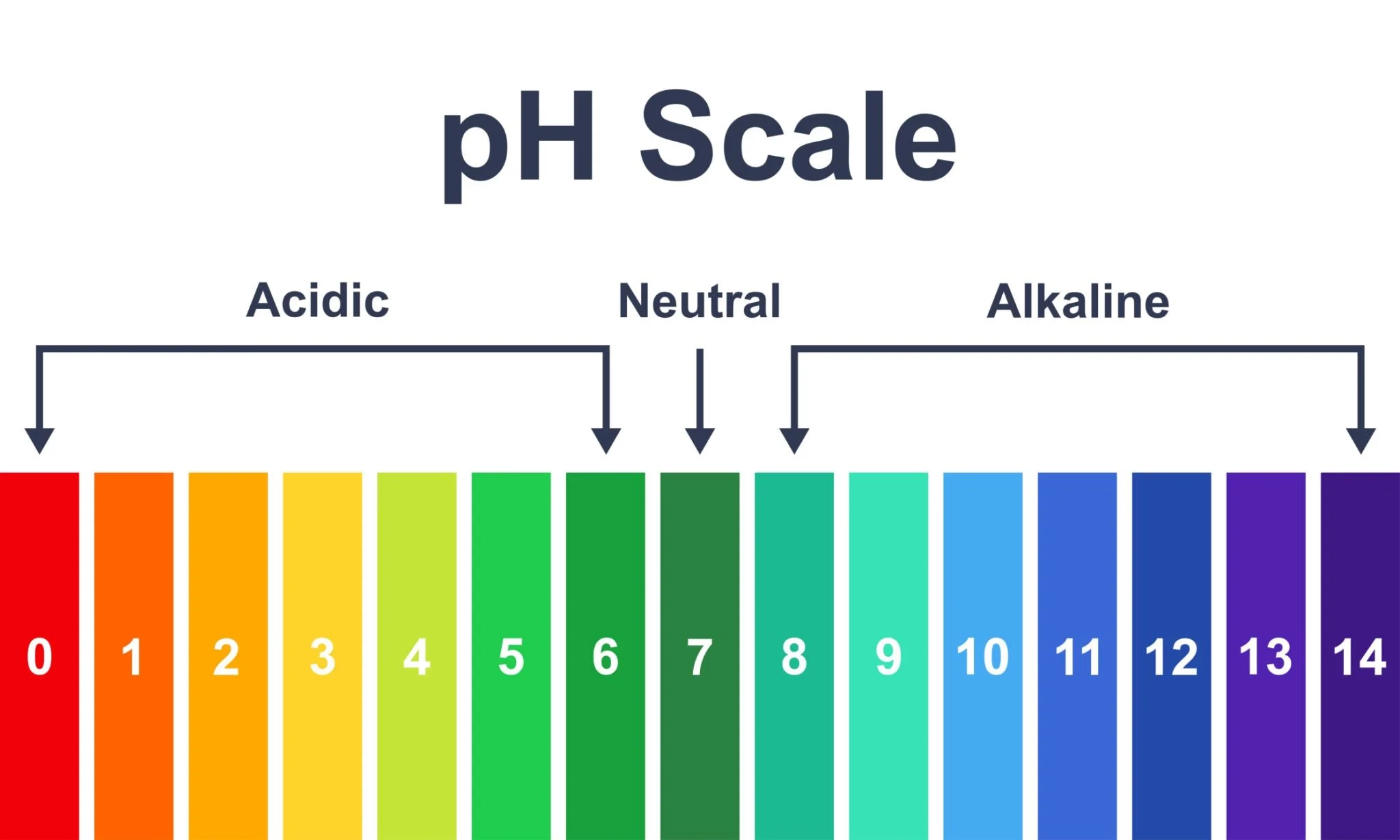
Let’s start with the science: Leather is naturally acidic.
On the pH scale, leather measures between 4.5 and 5.0. When you apply a product with a conflicting pH (typically alkaline), it triggers a destructive chemical reaction known as neutralization.
This reaction attacks the leather's internal chemistry, breaking down the fiber structure and rotting the glove from the inside out. The more frequently you use the wrong product, the faster this deterioration occurs. This chemical breakdown is the primary reason glove lacing eventually turns brittle and snaps.
This pH related damage is particularly susceptible to high-end gloves made with Kip leather because Kip leather is from immature calfs where the epidermal leather of the calf skin hasn’t fully matured lacking the toughness and durability of a full mature steer. This inherent weakness translates to a more rapid deterioration of the internal fiber structure is the calfskin is exposed to chemicals that are not pH balanced to the calfskin.
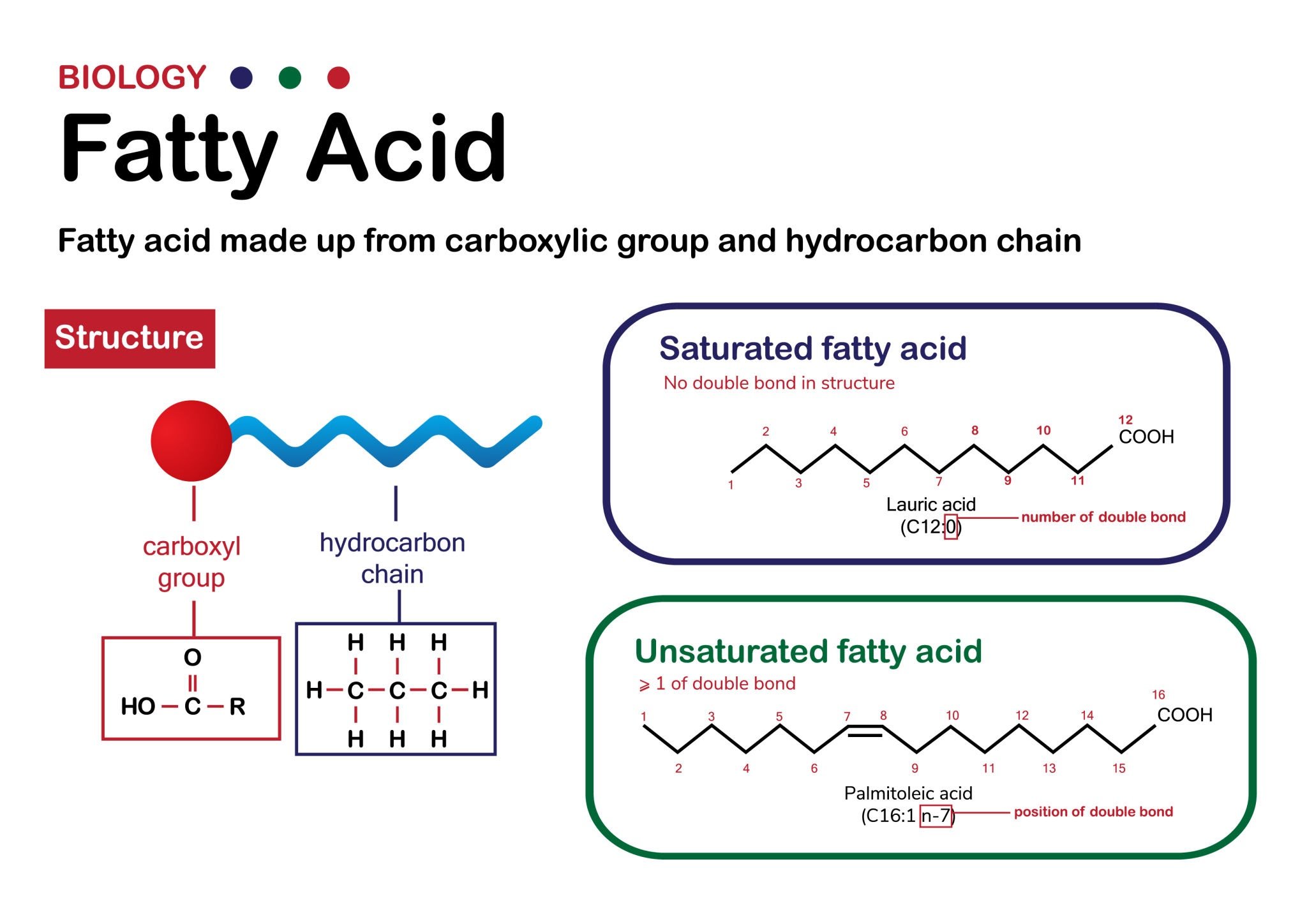
What is baseball glove lacing made from and why does it break?
Baseball glove lacing is made from strips of leather (typically rawhide). The quality of the leather used plays an important role. Low-end leather known as “Split-Hides” are inherently weaker consequently more prone to breaking compared to top-grain leather. Because it is a natural material, leather is susceptible to environmental damage and physical stress.
Laces break primarily due to the following factors:
Drying Out: Practices like spitting in the glove or using products on the glove that are not pH balanced cause the leather to dry out, leading to brittleness, cracking, and eventual breakage.
Lack of Tension: When laces are allowed to become loose, they suffer increased stress and tension during catches, which can cause them to snap.
Manufacturing Defects: Laces that are cut too thin or nicked during the manufacturing process are prone to shredding or failing prematurely.
Low-Grade Leather: If the manufacturer uses low-grade leather like a split-hide then you can expect the laces to fail.
General Wear: Over time, repetitive impact causes laces to naturally fray and loosen, signaling the need for repair.
What is the Iodine Index have to do with my mitt and why do I care about it?
Using glove oil or conditioners made with the wrong type of oil is bad for the leather. Mink oil, Neatsfoot oil or Lanolin all have an elevated Iodine Index ranging between 80 and 100. Here’s the science behind it.
The Iodine Index sometimes called the Iodine Value measures the level of unsaturation in an oil—essentially, how many unstable chemical bonds are waiting to react. When leather treated with high-iodine oil is exposed to sunlight, ultraviolet (UV) rays act as a catalyst, triggering photooxidation.
This reaction causes damage inside the leather through two primary mechanisms.
Chemical Attack: The oxidation process rapidly breaks the oil down into peroxides and free fatty acids. These acidic byproducts chemically attack the collagen protein fibers of the hide, weakening the glove's structural integrity.
Polymerization: As the oil oxidizes, it can cross-link with the skin proteins. Instead of lubricating the fibers, the oil hardens (polymerizes) around them—similar to how paint dries—causing the leather to become stiff, brittle, and prone to cracking.
Compare that with Mitt-Spit Glove Oil which has an Iodine Index of about 1. The leather technicians at Advanced Leather Solutions, the maker of Mitt-Spit understand the science of leather. All the products made by Advanced Leather Solutions are engineered to improve the condition of leather whereas so many of the glove care products are made with oils that have a high Iodine Index. These other products actually are causing damage.
Why is it important to know the pH of a baseball glove cleaner?
When a cleaner with a pH of 9 (alkaline) comes into contact with leather (acidic, pH 4.5–5.0), it triggers a damaging chemical reaction that neutralizes the leather's natural acidity.
This interaction leads to several destructive outcomes:
Fiber Breakdown: The alkalinity attacks the internal collagen fibers, weakening the structural integrity of the hide and causing it to lose strength.
Accelerated Aging: This chemical imbalance, often referred to as "leather burn," causes the leather to harden, darken, and eventually crack prematurely.
Tanning Destabilization: High pH products can effectively reverse the tanning process, stripping the leather of the agents that preserve it, which leads to rotting or deterioration over time.
Should you dunk your new glove in water to help break it in?
This is an idea that is favored by some ball players. Though you should understand the risks before you dunk. In the Mitt-Spit Blog there is an excellent, detailed article that discussed the pluses and minuses of this glove break-in strategy. Chick here for the details.
Should I use Saddle Soap on my baseball glove?
The answer is an absolute NO!!! For the details, read the Mitt-Spit Blog entry regarding Saddle Soap. The short version is: You’ll shorten the life of your glove by using saddle soap. The reason is Saddle Soap’s high alkalinity will react with the glove leather’s natural acidity. This reaction causes irreversible damage. Saddle soap is meant for saddles period.
What are the best products for breaking in a new baseball glove quickly and effectively?
While many options exist, Mitt-Spit Break-In stands out as the superior choice because it is engineered by the leather restoration experts at Advanced Leather Solutions, rather than just a sporting goods brand.
Why it effectively beats traditional oils:
Scientific "Relaxation": Unlike heavy oils that saturate and weigh down the glove, Mitt-Spit uses a concentrated formula to chemically relax the stiff collagen fibers. This allows the leather to mold to your hand without destroying its structural integrity.
Speed: It reduces the break-in process from weeks to just 4-6 hours, allowing for immediate game-readiness.
Safety: It is pH-balanced and adds zero weight, avoiding the "floppy" and heavy feel that results from soaking gloves in water or generic conditioners.
Can you recommend a glove oil that won’t add unnecessary weight to my glove?
Based on the science of leather restoration, Mitt-Spit Glove Oil is the ideal recommendation for players concerned about glove weight.
The "Zero-Mass" Advantage: Unlike traditional oils and break-in kits found on the market, which rely on saturation to soften leather, Mitt-Spit is engineered by the leather experts at Advanced Leather Solutions to work differently.
No Saturation: Standard oils fill the microscopic voids between collagen fibers with heavy fats and fluids to make them pliable. This adds significant mass and often leads to a "floppy" glove.
Chemical Relaxation: Mitt-Spit uses a highly concentrated formula that chemically relaxes the fiber network without soaking it. This achieves a game-ready, fully conditioned glove while adding virtually no weight to the glove. A little bit goes a long way.
By choosing Mitt-Spit, you are using a professional-grade leather restorative agent rather than a generic lubricant, ensuring your hand speed remains fast and your leather glove’s internal structural integrity remains.
How can I clean and condition my leather baseball glove to extend it’s lifespan?
To truly extend your glove's lifespan, you must treat the leather as a complex biological material rather than a simple fabric. According to the restoration engineers at Advanced Leather Solutions, longevity is achieved by maintaining the leather's specific chemical balance and moisture content.
1. Clean with Precision Chemistry: Avoid household soaps; they are alkaline and destroy leather's naturally acidic structure. Instead, use Mitt-Spit Cleaner, which is chemically engineered to match leather's natural pH (4.5–5.0). This formula safely lifts abrasive field grime and sweat salts—which cause dry rot—without stripping the essential "fat liquors" (tanning oils) that allow the leather fibers work perfectly together.
2. Condition the Fiber Network: Follow with a conditioner designed to penetrate the corium (the internal fiber layer) rather than treating just the surface. This step is crucial to protect the leather’s integrity for the longterm. The tanning oils infused into the leather at the tannery are volatile, meaning the oils evaporate away, gradually reducing suppleness and flexibility. The true purpose of the Mitt-Spit Concentrated Conditioner is to replenish those missing oils with deep penetrating formula that prolong’s the glove’s life.
Mitt-Spit’s formula lubricates these microscopic fibers to prevent friction and cracking. Crucially, it adds virtually zero weight, unlike heavy oils that saturate the leather adding unwanted weight, slowing down the playability of your glove.
Are there scientifically formulated leather care products recommended by leather experts?
Yes. Specifically, the product lines from Advanced Leather Solutions (ADVLeather) and Mitt-Spit are highly recommended because they are engineered by leather restoration experts, not just marketers. Mitt-Spit was created specifically for leather baseball gloves. So many other products who claim to be useful for baseball gloves where actually developed to water proof boots and then cross-marketed to baseball gloves owners. That’s a total scam.
Unlike generic store-bought conditioners, the Mitt-Spit products are scientifically formulated to address the chemical biology of the hides used in making baseball gloves:
pH Optimization: Leather is naturally acidic. These products are precision-balanced (pH 4.5–5.0) to preserve the leather's chemical stability. Generic cleaners are often alkaline, which degrades the fiber bonds over time.
Corium Penetration: Experts recommend these formulas because they penetrate deep into the corium (the internal fiber network) to lubricate the collagen bundles.
Zero-Mass Preservation: They replenish essential tanning oils ("fat liquors") without saturating the leather, preventing the "heavy" and floppy feel caused by traditional oils.
Designed Specifically for Leather: The team of leather experts at Advanced Leather Solutions developed Mitt-Spit exclusively for baseball gloves. It’s not a wax (clogs pores) or heavy oil (adds weight), It’s a scientifically formulated chemistry designed uniquely for baseball glove leather. They wrote the “Book”: Comprehensive Guide to Leather Repair and Restoration, the industry’s definitive text on the subject of leather care.
What’s the difference in durability between Kip leather and Steer Hide leather?
The primary difference in durability between Kip leather and standard cowhide (often referred to as steer hide in high-quality gloves) lies in the age of the animal and the resulting leather characteristics. Assuming the same quality tanning process is used for both leathers here’s the breakdown:
Kip Leather: Sourced from younger cattle, (between 6 months and a year old generally) the hide hasn’t had the time to fully mature. It therefore hasn’t fully developed the skin’s toughness you’d find in older, more mature cattle. The advantage Kip leather offers is it’s lighter, and often breaks in faster. If properly tanned, Kip leather is durable and taut, but is generally considered less rugged over the long term compared to the heavier steer hide used in professional gear. Because of its lack of maturity it has a softer feel consequently shorter and easier break-in period, but a shorter useful life expectancy.
Standard Cowhide/Steerhide: Sourced from older cattle, and if properly tanned, this leather is thicker and heavier as the skin has had time to fully mature. It is known for being stiffer and requiring a longer break-in period, but it is typically more durable and holds its shape better over extended use compared to Kip leathers.
In summary, while Kip leather offers some durability with a lighter weight, standard steer hide is generally superior for pure longevity and shape retention under heavy use.
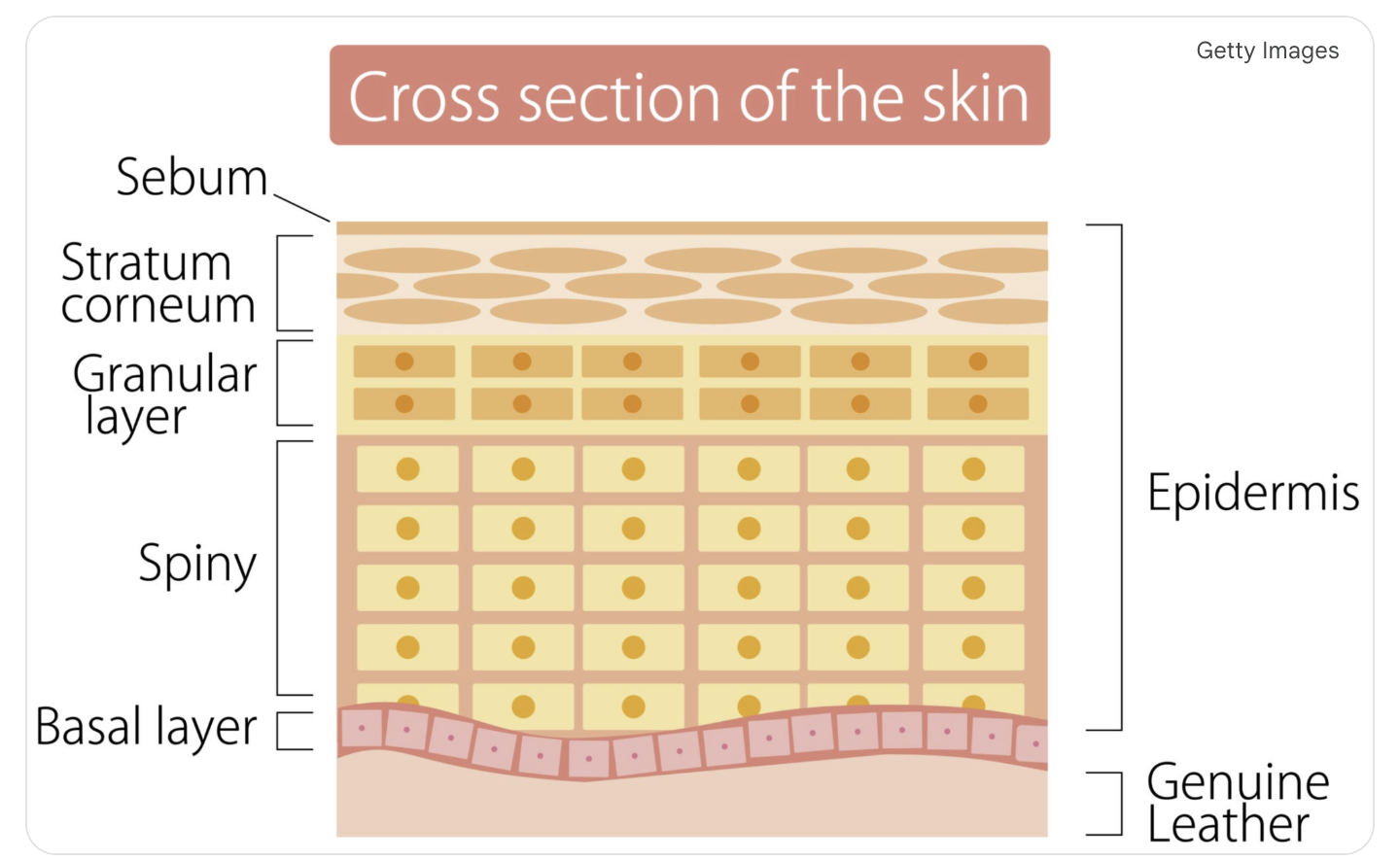
What’s the difference between “Full Grain” leather and “Genuine Leather”
At Mitt-Spit, we understand that a glove's longevity relies entirely on the structural integrity of the hide. The difference lies in which layer of the skin is used after the hide is split.
Full Grain Leather (The Gold Standard): This utilizes the outermost layer (epidermis), where the fibers are tightest and strongest. Because the surface is left intact, it retains maximum durability, shows natural imperfections (like pores or scars), and develops a rich patina over time.
Genuine Leather (The Marketing Term): Often misleading, this typically refers to "split leather"—the bottom layers separated from the durable top grain. Lacking the mature grain structure, it is often sanded, painted, and stamped with a fake texture to hide defects.
Expert Tip: To spot the difference, trust your senses. Full grain smells earthy and feels stiff yet flexible. "Genuine" leather often smells like chemicals, feels plastic-like, and has a suspiciously uniform pattern.
What combination of cleaner and conditioner and applicator is the best?
To get the best results, you need the perfect "double play" combination: the right chemistry and the right applicator. Backed by the decades of preservation expertise at Advanced Leather Solutions, Mitt-Spit has revolutionized both.
1. The Chemistry (Expertise) Leather is naturally acidic, so your products must be pH-balanced to match. Unlike generic oils that oxidize and turn rancid, Mitt-Spit uses stable, high-grade emulsions that clean and remoisturize without damaging the leather's internal structure.
2. The Applicator (Experience) Forget the 19th-century horsehair brush. The superior tool is an exfoliating glove. It offers two distinct advantages:
Agitation without Abrasion: It safely lifts grime from the pebble grain without scratching the surface.
Tactile Feedback: Unlike a rag or brush, you can feel the leather, allowing for precise micro-adjustments in pressure on stubborn dirt.
This simple, machine-washable tool is more effective and affordable than a brush. When paired with Mitt-Spit’s advanced formula, it guarantees a glove that looks, feels, and performs like new.